Balance K₂Cr₂O₇ + HCl Using Oxidation Number Method | Redox Reaction | JEE Chemistry Doubtify
🔥 Balance Redox Reaction Using Oxidation Number Method | JEE Chemistry | Doubtify
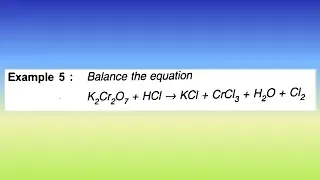
⚛️ Question :
Balance the following equation by the oxidation number method:
K₂Cr₂O₇ + HCl → KCl + CrCl₃ + H₂O + Cl₂
📘 Solution:
This is a redox reaction where potassium dichromate reacts with hydrochloric acid to produce potassium chloride, chromium(III) chloride, water, and chlorine gas.
Let’s balance it step-by-step using the oxidation number method:
🔬 Step 1: Assign oxidation numbers
Reactants:
-
K₂Cr₂O₇: Cr has oxidation number +6
-
HCl: Cl has oxidation number –1
Products:
-
CrCl₃: Cr is +3
-
Cl₂: Cl is 0
So here’s what’s happening:
-
Cr is getting reduced: +6 → +3 (Reduction)
-
Cl is getting oxidized: –1 → 0 (Oxidation)
🔁 Step 2: Write the changes in oxidation numbers
-
Chromium (Cr): 2 atoms × change of 3 = total change = 6
-
Chlorine (Cl): x atoms × change of 1 = total change = x
So to balance the changes:
6 electrons lost = 6 electrons gained
⇒ x = 6 Cl⁻ ions oxidized
So we need 6 Cl⁻ to be oxidized, which means 3 Cl₂ molecules will be formed (since each Cl₂ has 2 atoms).
🧪 Step 3: Write the partial balanced redox equation
Unbalanced skeletal:
K₂Cr₂O₇ + HCl → KCl + CrCl₃ + H₂O + Cl₂
Now, we use the changes in oxidation number to balance the atoms.
⚖️ Step 4: Balance atoms one by one
-
Cr atoms:
K₂Cr₂O₇ → 2 CrCl₃
(Already 2 Cr atoms on both sides) -
Cl atoms:
From KCl, CrCl₃, and Cl₂-
2 KCl → 2 Cl
-
2 CrCl₃ → 6 Cl
-
3 Cl₂ → 6 Cl
⇒ Total = 2 + 6 + 6 = 14 Cl
So, we need 14 HCl on reactant side.
-
-
K atoms:
2 KCl ⇒ 2 K → So we need 1 K₂Cr₂O₇ -
H and O atoms:
From 14 HCl → 14 H
7 H₂O → 14 H and 7 O
⇒ Balance with 7 H₂O
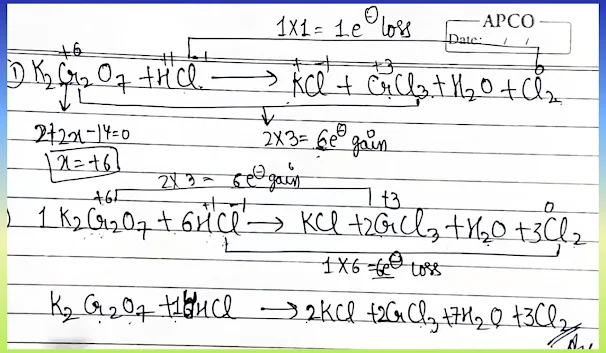
✅ Final Balanced Equation:
K₂Cr₂O₇ + 14 HCl → 2 KCl + 2 CrCl₃ + 7 H₂O + 3 Cl₂
🧠 Solution Image:
🎯 Why This Question is Important
-
It's a classic example of balancing redox reactions using oxidation number method, a very important topic in JEE Mains and Advanced.
-
Helps build strong conceptual clarity on oxidation, reduction, and electron transfer.
-
Strengthens understanding of stoichiometry, valency, and chemical reaction mechanisms.
-
Often seen in both CBSE board exams and competitive entrance tests.
🧠 Pro Tip:
Always identify elements undergoing oxidation and reduction first. Match total increase and decrease in oxidation numbers to keep electron balance perfect.
🎥 Video Solution:
📩 Have a Doubt?
Message us on Instagram or Email at doubtifyqueries@gmail.com – we reply within 24 hours!



Comments
Post a Comment
Have a doubt? Drop it below and we'll help you out!